1. Overview
PMAnti-acne® 9997 is a combination of magnolia officinalis bark extract, pueraria lobata root extract and glycyrrhizinic acid. One of the common Chinese medicines is Houpu (magnolia officinalis), the Chinese medicine Houpu refers specifically to the dried dried bark, root bark and branch bark of the plant. It has the efficacy of removing dampness, warming the body and relieving pain, and calming asthma. The decoction of Houpu has strong antibacterial effects on staphylococci and streptococci, etc. Pueraria lobata is one of the most common Chinese medicines in China, with a long history of clinical use. 1959, Shibata et al. reported for the first time that puerarin is a unique constituent of Pueraria lobata; and Song et al. have provided a detailed overview of the studies on the chemical constituents of pueraria lobata that were published prior to 2014. The main compounds are flavonoids, triterpenoids, coumarins and saponins. Studies have shown that Pueraria lobata has the functions of promoting cardiovascular and retinal blood flow, inducing cancer cell differentiation, hypoglycaemic, hypotensive, antioxidant, antipyretic, improving memory and enhancing immunity. It has been found by Plamed that Pueraria lobata has a strong inhibitory effect on 5α-reductase; Glycyrrhizinic acid, also known as glycyrrhizinic acid, is the main active ingredient of the natural plant Glycyrrhiza glabra, a classical anti-inflammatory drug, and an important raw material for cosmetics. Glycyrrhizinic acid is both anti-inflammatory and anti-allergic, inhibits bacterial reproduction. Also, it relieves the toxic side effects of cosmetics and other external factors on the skin. In addition, it can effectively inhibit the activation of tyrosinase and prevent the production of melanin, with certain whitening effect. At present, it is widely used in medicine, cosmetics and other fields.
Therefore, PMAnti-acne® 9997 is a natural cosmetic ingredient specially researched and promoted by Plamed to eliminate acne, also with oil control, anti-inflammatory and whitening effects.

| Product Name | INCI Name |
| PMAnti-acne® 9997 | MAGNOLIA OFFICINALIS BARK EXTRACT |
| PUERARIA LOBATA EXTRACT | |
| GLYCYRRHETINIC ACID | |
| HYDROXYPROPYL CYCLODEXTRIN | |
| Minimal Package | Minimal Order Quality |
| 1kg | 1kg |
2. Botanical Source
Houpu, Chinese medicine name, is for the Magnolia family Magnolia genus Magnolia dry bark, root bark and branch bark. It originates from China’s Sichuan and Hubei and other places, with the continuous improvement of planting technology, its medicinal value is also very high, so in some of China’s northern regions or other places have also begun to use modern herbal planting technology to start cultivating and planting, but also, now in the influence of traditional Chinese medicine is also gradually increased, the value of Chinese herbal medicine is also gradually being valued.
Pueraria lobata, the name of Chinese medicine, is the leguminous plant kudzu dry root. China’s most areas of production, mainly distributed in Liaoning, Hebei, Hubei, Guizhou, Yunnan, Shanxi, Shaanxi, Gansu and other places. Pueraria lobata is a medicinal plant, in China’s folk history of more than 3,000 years. As a valuable Chinese medicinal herbs, it has been thousands of years of long history. It is an important herb for clearing heat and removing toxins, and caring for the liver and kidneys through the ages. It has been clearly documented in dozens of literature such as Shennong’s Classic of the Materia Medica, Compendium of Materia Medica and Chinese Pharmacopoeia.
Licorice (Glycyrrhiza uralensis Fisch.), common name: sweet root, sweet grass, etc., legumelicorice perennial herbaceous plants, national second-class protected plants, “the king of medicines” reputation. Licorice has been recorded in Chinese herbal books throughout the ages, it has good efficacy on the treatment of chronic bronchitis, wheezing bronchitis. In addition, liquorice can also detoxify all kinds of medicines, which means “detoxification” to the Chinese people, and because it can harmonise all kinds of medicines, it has the symbolism of “harmonisation and mediation”.
3. Technical Data Sheet
| ANALYSIS | SPECIFICATION |
| Magnolol | ≥15% HPLC |
| Pueraria flavones | ≥5% UV |
| Glycyrrhizinic acid | ≥6% HPLC |
| Organoleptic Tests | |
| Appearance | Powder |
| Color | Off-white |
| Odor | Characteristic |
| Physical Characteristics | |
| Moisture | ≤10.0% |
| Ash content | ≤7.0% |
| Heavy Metals | |
| Pb | ≤10ppm |
| As | ≤2ppm |
| Hg | ≤1ppm |
| Cd | ≤5ppm |
| Microbiological Tests | |
| Total of bacteria | ≤1000cfu/g |
| Yeast & mold | ≤100cfu/g |
| Pseudomonas aeruginosa | Not detected |
| Staphylococcus aureus | Not detected |
| Heat-resistant escherichia coil | Not detected |
4. PMAnti-acne®9997 Mechanism & Function
- Anti-acne
- Minimum inhibitory concentration
This test is based on the 2002 version of the Ministry of Health’s “Disinfection Technical Specification” – 2.1.8.3 Minimum Inhibitory Concentration (MIC) test. Use the agar dilution method, dissolve a mixture of different concentrations of bacteriostatic agents in agar medium, and then seed Propionibacterium acnes. Through the growth of bacteria or not, determine the minimum concentration of anti-bacterial substances to inhibit the growth of tested bacteria. i.e., the Minimal Inhibitory Concentration (MIC). The test results for sample PMAnti-acne® 9997 are shown below:
 The minimum inhibitory concentration of PMAnti-acne® 9997 against Propionibacterium acnes (ATCC 11827) was 0.03mg/mL.
The minimum inhibitory concentration of PMAnti-acne® 9997 against Propionibacterium acnes (ATCC 11827) was 0.03mg/mL.
- Carrier immersion quantitative bacteriostatic test
WS/T650-2019 antibacterial and bacteriostatic effect evaluation method 5.1.2 Carrier immersion quantitative bacteriostatic test. The test results of the sample PMAnti-acne® 9997 are as follows:
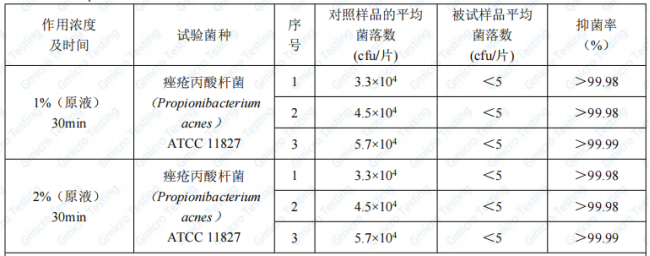
After testing, with 1% and 2% concentration of PMAnti-acne® 9997 were treated for 30min, the test was repeated 3 times. The bacteriostatic rate of propionibacterium acne (ATCC 11827) was >90%, which met the requirements of WS/T650-2019 “Antibacterial and Bacteriostatic Effect Evaluation Method” (bacteriostatic rate) ≥90%. It has strong bacteriostatic effect on the tested strains.
- Anti-acne test on human
In this study, a part of qualified subjects were recruited to use the test product (containing 1% PMAnti-acne® 9997 basic formula emulsion) for a certain period of time to evaluate the acne efficacy of cosmetics by means of instrument testing and visual evaluation. The test results are as follows:
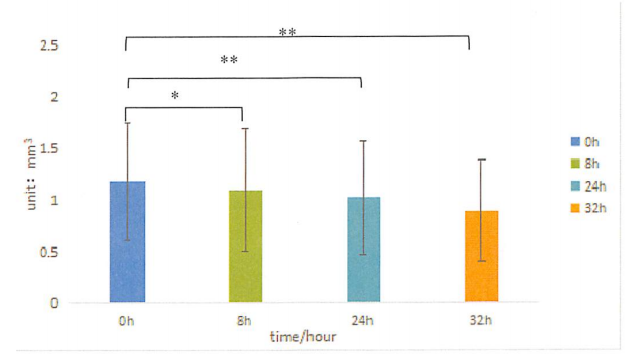 Figure. Acne volume before and after use
Figure. Acne volume before and after use
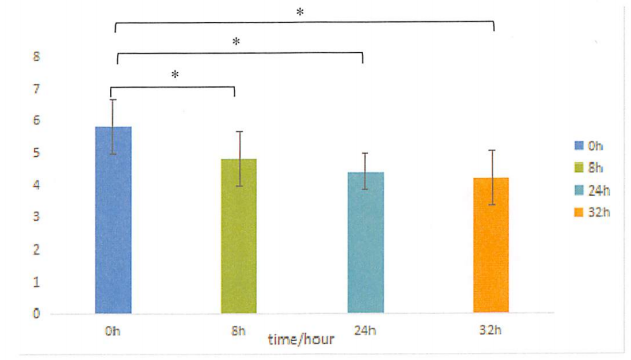
Figure. Acne number before and after use
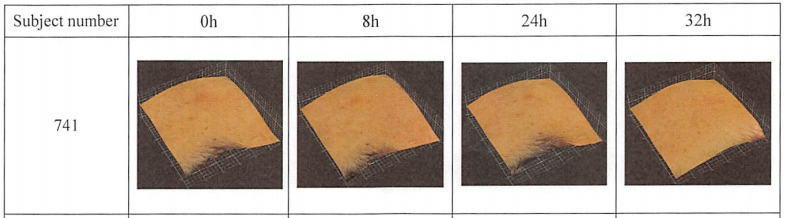 Figure. Shows effective example
Figure. Shows effective example
The test results showed that at 8h, 24h, and 32h, the number and volume of acne in the test sample area were significantly different from the baseline value, demonstrating that PMAnti-acne® 9997 has anti-acne efficacy.
- Oil Control
- 5-α reductase inhibition rate test
5α-reductase is an important androgen metabolising enzyme in the skin. It irreversibly converts testosterone (T) to dihydrotestosterone (DHT), the most active androgen, which induces the sebaceous glands to overproduce oil. Therefore, lowering the level of DHT by inhibiting 5α-reductase activity can effectively alleviate the overproduction of sebum by sebaceous glands.
The samples PMAnti-acne® 9997’s test results as below:
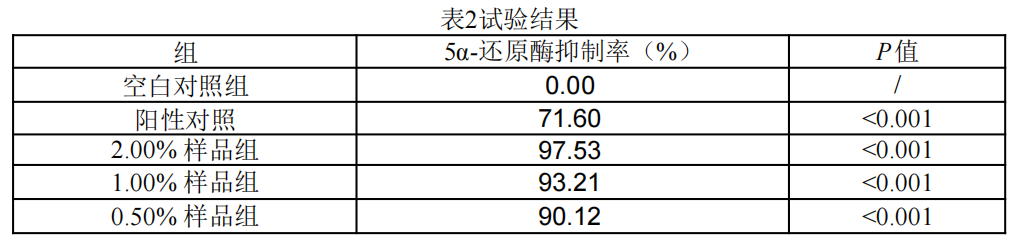
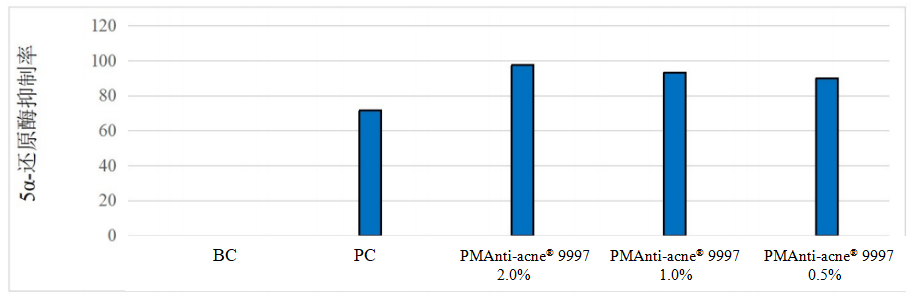
Figure. PMAnti-acne® 9997’s test results to inhibit 5α-reductase
Test conclusion: At concentration of 0.5%, 1% and 2%, the inhibition rate of PMAnti-acne® 9997 on 5-α reductase was 90.12%, 93.21% and 97.53%, respectively, indicating it has oil control effect.
5. PMAnti-acne® 9997 Advantages
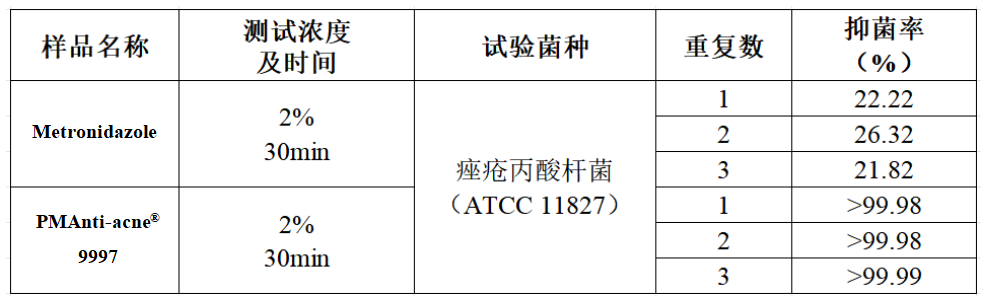
- Outstanding acne efficacy, Plamedbased on the inhibition of Propionibacterium acnes test found that PMAnti-acne® 9997 inhibition rate of >99.9%, while the common anti-acne raw material metronidazole inhibition rate of about 20% or so (the standard judgement of non-inhibition of bacteria), the same dose of PMAnti-acne® 9997’s bacterial inhibition is far more than metronidazole :
- Strong oil control effect, PMAnti-acne®9997 inhibition rate of 5α-reductase is up to 97.53%.
6. PMAnti-acne® 9997 Solubility
PMAnti-acne® 9997’s maximum solubility in butanediol 70% (temperature 70℃)
7. PMAnti-acne® 9997 Dosage
| Application | Recommended Dosage |
| Lotion, serum, essence, cream, etc. | 0.5%~5.0% |
8. PMAnti-acne® 9997 Safety
- Magnolia Officinalis Bark Extract, Pueraria Lobata Extract, And Glycyrrhetinic Acid are all listed in Announcement on the List of Names of Used Cosmetic Raw Materials issued by China NMPA in 2014.
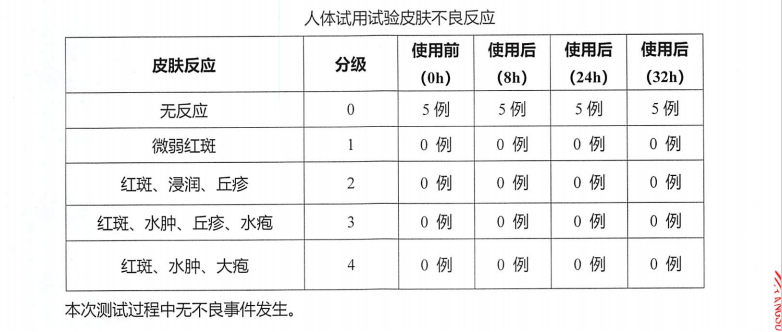
- During the human efficacy test, subjects had no adverse skin reactions after 32h of use of PMAnti-acne 9997.
9. Packing and Storage
Package: Packed in 1kg aluminum foil bag or 25kg paper drums with two plastic bags inside.
Storage: Stored in a cool and dry place and away from direct sunlight and oxidizing agents.
10. Related reading
Plamed focuses on natural cosmetic ingredients for more than 10 years. We have founded four subsidiary companies, which respectively develops different kinds of cosmetic raw material. Plamed is a company whose CEO is designated as the first secretary general of Shaanxi Plant Extraction Association.
As a professional anti-acne ingredient PMAnti-acne® 9997 manufacturer, Plamed have been constantly upgrading the production process. We firmly believe that good anti-acne ingredient PMAnti-acne® 9997 and good anti-acne ingredient PMAnti-acne® 9997 price will help customers make good terminal products and help customers win a lasting and broad market.













